Medical Devices Assembly
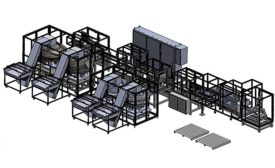
Automated Assembly System Roundup
Auto parts, plumbing products and medical devices are among the myriad items made on automated assembly systems
October 4, 2019
Optimizing Ultrasonic Plastic Welds
This advice should assist you in solving day-to-day ultrasonic welding challenges
October 2, 2019
Integrating Presses Into Assembly Systems
As the stand-alone, product-dedicated press becomes more obsolete, workcells and assembly lines with integrated presses are becoming more flexible and productive
September 9, 2019
Joint Design for Ultrasonic Welding
These tips will help you design plastic parts for optimal assembly with ultrasonics
September 6, 2019
Never miss the latest news and trends driving the manufacturing industry
Stay in the know on the latest assembly trends.
JOIN TODAY!Copyright ©2025. All Rights Reserved BNP Media.
Design, CMS, Hosting & Web Development :: ePublishing