Designing a medical device is more complicated than designing a toaster or an automotive cooling system. Besides the issues common to any product—feasibility, usability, and design for manufacture and assembly—there are also issues of biocompatibility, sterilization and FDA regulations to deal with.
It’s enough to overwhelm even experienced engineers. That’s why some manufacturers turn to outside design firms for help. Such firms aren’t merely for inventors who need help with an idea hastily sketched on a cocktail napkin. In fact, even major manufacturers occasionally seek help from design firms, even if it’s just to get a fresh perspective on an existing product.
This was the case a few years ago when AbbVie Inc. set out to design a new auto-injector pen. The pharmaceutical giant retained Chicago-based firm Design Integrity Inc. to lead the industrial design and preliminary mechanical engineering effort.
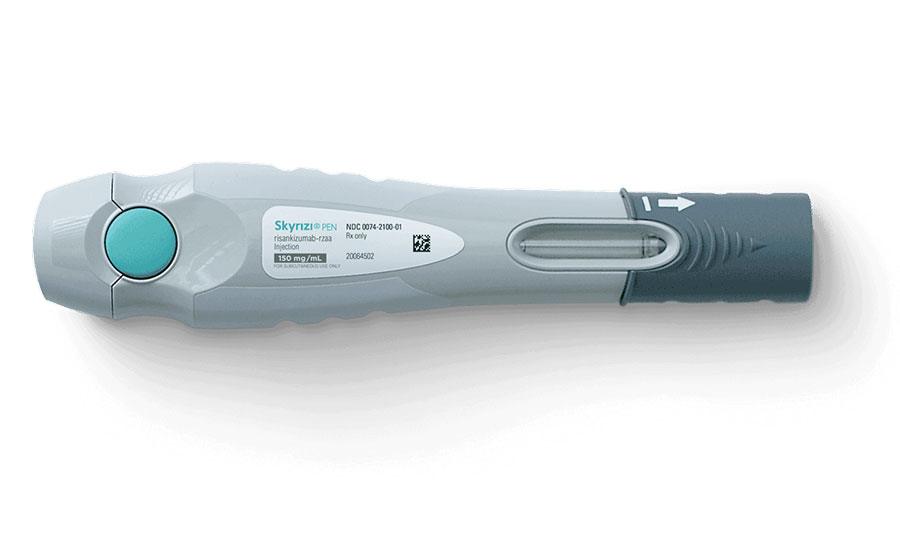
This auto-injector pen was developed to be easy to use and to reduce the burden on patients when performing self-injection. Photo courtesy AbbVie Inc.
Throughout the project, Design Integrity’s team developed innovative designs for various aspects of the device, including the form language, user interface and overall construction. The new device was launched internationally by AbbVie, and it has been lauded for its ease-of-use.
AbbVie’s existing delivery device was well-received in the marketplace, but it was not unique to the AbbVie product line. When planning for the next gen auto-injector, AbbVie strove to improve the aesthetic design as well as the usability for both women and men with a wide range of hand sizes, including a subset of the population with compromised physical dexterity. To reduce the time to market, an existing internal mechanism was used, but the new outer housing subassembly had to be developed from the ground up.
The industrial design team at Design Integrity began with a thorough assessment of market and user research data previously compiled by the team at AbbVie, followed by a detailed design assessment of the existing pen. As the project goals included improved ergonomics and ease of use for a wide variety of users, anthropometric data for human hand sizes was studied in detail. Next, a broad range of preliminary design concepts was developed, focused primarily on ergonomic hand grip forms and interface aesthetics.
After a review of the initial design concepts with the team at AbbVie, several leading directions were selected for further development. Next, a series of scaled study models were hand-carved from foam for in-hand evaluation by male and female team members at AbbVie and Design Integrity.
After the initial reviews of the foam models, each model was refined with improvements to the aesthetic form and grip areas to improve comfort and usability. The team reviewed the refined foam models along with the concept sketches and renderings, and an intended design direction was selected.
The chosen design direction was refined extensively, including an extensive exploration of grip locations and forms. In parallel, a color study was conducted to evaluate potential colors for the plastic housing components, activation button, and over-molded grips. Once the preferred grip and button design layout was approved, the project plan was solidified for the preliminary mechanical design effort.
A preliminary 3D CAD layout of the new auto-injector housing was developed with SolidWorks 3D CAD software. The outer geometry was developed first, using the final foam study model as the reference to ensure that critical sizes and design features were accurately incorporated in the CAD model.
Once the outer form was completed, the housing components were “shelled” by offsetting the outer surfaces by the intended wall thickness of the housings. Preliminary mounting features were added to the inside surfaces to position and retain the pen’s internal mechanism.
The grip features were then added to the CAD model, and the walls of the housings were adjusted below the grip areas to ensure a consistent nominal wall thickness across the main surfaces of the plastic components. From the onset, the 3D CAD design was developed with production intent.

AbbVie’s new injector pen was designed from the outset with production in mind. Photo courtesy AbbVie Inc.
After the initial 3D CAD layout was completed, a weighted prototype was fabricated for evaluation. The plastic housings were CNC machined from ABS plastic blocks, and the grip areas were cast onto the plastic housings at a local shop. The plastic housings were painted a neutral, light gray color. The goal for the prototype was to allow for the evaluation of the overall form, aesthetics, and ergonomics (grip form, comfort and button location).
Following the evaluation of the scale prototype, the Design Integrity team refined the design of the auto-injector handle in 3D CAD with a high level of production intent. The design files were then handed off to the AbbVie team for further prototyping, usability testing, engineering testing, final design refinements, and production implementation.
The new auto-injector pen was launched internationally by AbbVie in 2018, and the design was well-received in the marketplace. According to AbbVie team members, the handle design performed better during user studies than all prior injection devices.
The new auto-injector pen was developed to be easy to use and to reduce the burden on patients when performing self-injection. The body of the injector pen fits in the hands of a wide variety of patients, including individuals with a weak grip or reduced physical dexterity.
The pen allows users to complete a full injection in about 10 seconds. In addition, the housing form conceals the needle tip when injected. Overall, the project was a big success, and the device was launched across several international markets.
Editor’s note: For more information on design firm services, visit www.designintegrity.com. Phil Anthony will be giving a presentation, “Design Firms—How They Can Help Your Company,” at The ASSEMBLY Show on Thursday, Oct. 24.
Learn about new products for medical device assembly
New Generation Collaborative CVGC Carbon Vacuum Gripper
Precision Link Conveyors and Medical Manufacturing
Provide safe, high quality care with ACE






