Medical Device Manufacturer Uses 3D Printing to Prototype Parts

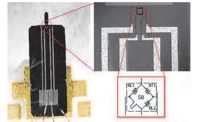
In 2018, HemoSonics had Proto Labs develop a prototype casing for the company’s Quantra System blood-clot analysis machine. Photos courtesy Proto Labs Inc.

Proto Labs’ services include 3D printing, CNC machining, injection molding and sheet metal fabrication. Photo courtesy Proto Labs Inc.


For various reasons, the market for portable medical devices continues to significantly grow. Researchers say the global market hit nearly $47.73 billion in 2021, and project it to increase to about $77 billion by 2028.
A key segment of the market is hemostasis diagnostic devices, which ensure blood fluidity and blood vessel integrity. Last year, the market for these devices reached $4.1 billion. It is expected to reach $8.57 billion in 2026.
Charlottesville, VA-based HemoSonics LLC has been making a mark for itself in the hemostasis device market since 2005. That year, founders Bill Walker, Mike Lawrence, and Francesco Viola introduced an ultrasound-imaging method to measure the stiffness of blood clots.
Over the next 13 years, the company secured key patents, conducted numerous hospital studies, and consulted with physicians and other clinicians. It also established a working relationship with rapid prototyping firm Proto Labs Inc. (PL).
Since 2012, PL has provided thousands of key machine parts and hundreds of prototypes quicker than any supplier HemoSonics could find. The parts and prototypes have been produced through 3D printing, CNC machining, injection molding and sheet metal fabrication. Their uses range from robotic fixturing to thermal control units and pneumatic manifolds.
The latest HemoSonics-PL collaboration occurred in late 2018 when HemoSonics engineers needed prototype parts about the size of a computer monitor for the casing around its Quantra System blood-clot analysis machine. The parts were required to demonstrate the form, fit and function of the system to physicians at various hospitals.
PL initially created the casings using 3D printing, before injection molding them. The latter process presented one big challenge to PL: making the casings a Pantone color that exactly matched the HemoSonics marketing department’s specifications.
Normally, PL injects a 3 percent salt-and-pepper mix of plastic colored resins that are the natural color of the specific material into the mold. Doing this usually results in final parts that are very close to the preferred color.
In this case, however, some swirling and flow marks showed up on the first batch of HemoSonics’ casings. PL then collaborated with one of its plastic resin suppliers and HemoSonics to pre-compound the colors. The plastic resins were mixed with a unique dye before molding to ensure that the pellets had a nice uniform color.
On the material side, HemoSonics required Quantra be made of ABS plastic that meets strict flammability and durability standards. Company engineers also heat-stake and pad-print the injection-molded casings. The heat staking melts metal threaded inserts into the parts so screws can easily attach the casing to the Quantra frame. HemoSonics uses pad printing to apply its company logo to each casing.
After several months of showcasing the encased Quantra to doctors in Europe, HemoSonics received the CE Mark, allowing for continentwide distribution. In March 2019, the company announced that the product (and its initial QPlus cartridge) were granted marketing authorization by the FDA for U.S. distribution.
PL was founded as the Protomold Co. Inc. in 1999 before adding 3D printing, CNC machining and metal fabrication over the next 20 years. During the coronavirus pandemic, PL has produced countless face shields, plastic clips and components for coronavirus test kits used in Minnesota hospitals, and collaborated with the University of Minnesota to produce parts for a low-cost ventilator.
For more information, call 877-479-3680 or visit www.protolabs.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!