Additive Manufacturing for Medical Device Production
3D Printing Goes from Prototyping to Production

Additive manufacturing enables engineers to print a wide variety of prosthetic medical devices. Photo courtesy of AxisLab 3D Printing

This 3D-printed sternum is being prepped for implantation. Photo courtesy CSIRO

Physicians can print detailed anatomical models, such as this liver, directly from patient scans. By creating textures ranging from hard bone to soft tissue, healthcare professionals can plan, practice and determine therapy approaches or surgical techniques. Photo courtesy Stratasys Ltd.

This wearable, noninvasive device monitors electrical activity in the stomach over 24-hour periods. The 3D-printed plastic box, which encases a battery and electronics, is connected to 10 small electrodes. Photo courtesy University of California San Diego

These guides for ACL knee surgery were printed with Inconel 718 using direct metal laser sintering. Photo courtesy Stratasys Direct Inc.

These 3D-printed tracheal splints for babies suffering from a congenital breathing condition were made out of polycaprolactone. Photo courtesy University of Michigan

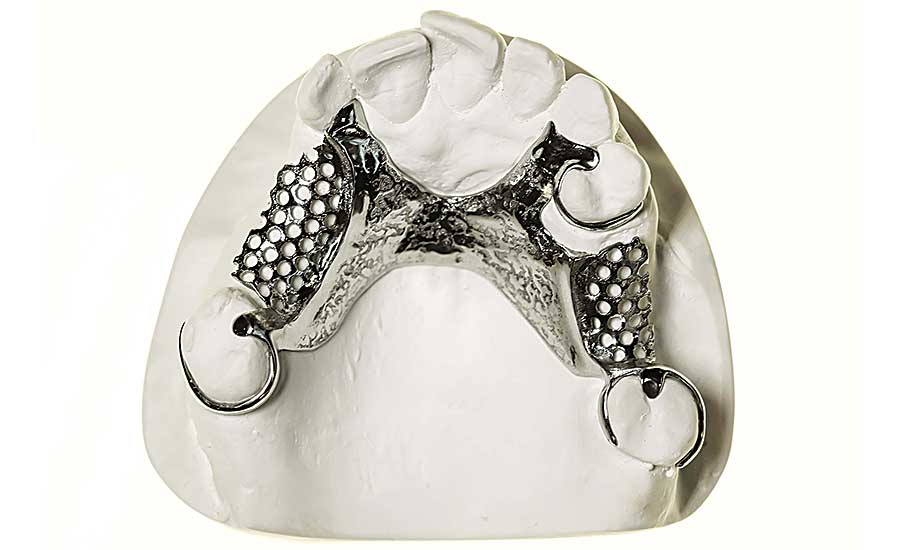
The dental industry has jumped on the additive manufacturing bandwagon to produce dentures and other devices. Photo courtesy Renishaw pl







Additive manufacturing is the hottest thing to hit the medical device industry since the first pacemaker was implanted in a patient 60 years ago. The technology has transformed the way that engineers design numerous products. Now, it’s starting to move beyond prototyping into production.
Medical device manufacturers are using 3D printing to create products that were previously impossible to make. Other products can be personalized to a specific patient or treatment. Engineers can create parts from plastic, metal, ceramics, silicone and other materials.
Additive manufacturing “prints” solid objects from a digital file by depositing one layer of material on top of another, rather than starting with a solid piece of material that is cut and shaped. It allows companies to more easily manufacture complex shapes and structures that have typically been difficult to make with traditional plastic-injection molding or machining methods. There’s also less waste, which results in shorter setup times and lower material costs.
Plastic printed part are usually made using ultraviolet, infrared or visible light in conjunction with laser or heat energy. Metal parts are produced with laser-based or electron beam-based printers that use metal powders for raw material; the laser or electron beam fuses together the powder.
In the medical device industry, 3D printing enables manufacturers to create customized products that cater to individual patient needs. Engineers are using the technology to produce a wide variety of products, including fixtures, guides, hearing aids, prosthetics, surgical tools, orthopedic implants and anatomical models for pre-surgery applications.
"Printing has significant advantages over traditional manufacturing methods, particularly for biomedical applications," says Keith McLean, Ph.D., director of CSIRO Manufacturing. "It allows for advanced personalization of implants so they uniquely fit their recipients, as well as rapid manufacturing, which could mean the difference between life and death for a patient waiting for surgery."
The dental industry has also jumped on the additive manufacturing bandwagon. In fact, demand for applications ranging from dentures to orthodontic aligners is expected to skyrocket over the next decade.
“Today, we’re seeing the largest and most rapid growth in the dental sector,” says Steven Pollack, senior staff research scientist at Carbon Inc., a leading digital 3D manufacturing company based in Silicon Valley. “By taking a scan of a patient’s mouth, oral surgeons and orthodontists can generate a variety of 3D objects that are useful for planning and performing various types of procedures."
In addition, 3D printing has spawned a new trend called point-of-care manufacturing. Many large hospitals and medical centers are implementing additive manufacturing labs that can create a variety of products in house, such as surgical tools, experimental heart valves and bone implants for use in clinical studies.
A Bold New Era
Some surgeons have already saved infants born with life-threatening breathing conditions by creating patient-matched 3D-printed splints. The devices expand and degrade as the babies grow.
“Additive manufacturing is gaining considerable momentum in the medical device industry on several fronts,” says Cory Forbes, vice president and chief technology officer at Nypro, a division of Jabil Inc. that provides design and manufacturing services for customers in the diagnostic, medical device, pharmaceutical and consumer health markets. It operates 23 facilities around the world that assemble a wide variety of medical devices, such as epinephrine autoinjectors, inhalers, kidney dialysis machines and scanners.
“Increasingly, 3D printing is being used in the production of end-use parts for standalone medical devices, along with patient-unique products and point-of-care solutions,” claims Forbes. “With patient-unique parts, such as dental aligners, additive manufacturing is one of several manufacturing methods used in final production.
“An emerging trend is to use additive manufacturing to print aligners directly without needing to rely on additional manufacturing methods,” explains Forbes. “This will enable dentists to deliver very precise solutions to patients in days, instead of the weeks it currently takes.”
“We [see] two tremendous growth opportunities,” says Nick Cordaro, CEO of Watershed Idea Foundry, a hybrid incubator and accelerator for the biomedical industry. “One is with any medical device requiring bone-on-growth or bone-in-growth, due to the osteoconductive nature of 3D-printed titanium. The second is related to fabrication of surgical tools using near net stainless steel components.”
“In audiology, approximately 98 percent of hearing aids are now manufactured with 3D printing technology, and the rejection rate is way down,” adds Lee Dockstader, director of vertical market development for 3D printing at HP Inc. “Hearing aids are more comfortable now because digital impressions of the patient’s ears are taken first, and the devices are printed based on each person’s unique ear shape and size.”
Increasingly, medical device manufacturers are taking advantage of the time and cost savings of additive manufacturing to 3D-print a variety of conventional parts, such as levers and brackets, which are needed for a variety of applications.
“For instance, point-of-care patient testing solutions can benefit greatly from additive manufacturing,” Forbes points out. “[Engineers can combine] multiple components, including tunnels, manifolds, fluidic chambers or other housings, without the production and assembly limitations typically imposed by conventional injection molding and tooling methods.
“When design for manufacturability constraints are removed, the possibilities to streamline and miniaturize overall product packaging grow exponentially,” claims Forbes. “Consider the emerging home healthcare market, where compact form factors are critical. With additive manufacturing, it’s possible to combine multiple components to reduce device sizes considerably, which is expected to drive significant growth in this application area.
“Medical devices that incorporate cartridges or manifolds that guide fluids typically are very complex, and therefore, ideally suited for additive manufacturing,” explains Forbes. “Not only are there many components that comprise these parts, there’s usually a need for laser or ultrasonic welding during assembly. With additive manufacturing, there’s an opportunity to produce lower-cost, improved part designs with fewer steps, while eliminating difficult processes altogether.”
Low-Volume Production
Another growing trend is the use of 3D printing to produce low-volume, complex parts for medical devices.
“Today, it’s extremely difficult to make and assemble complex parts containing a lot of pieces or unique geometries using traditional injection molding or machining technologies,” says Forbes. “With additive manufacturing, however, there’s limitless flexibility to design complex parts, as well as combine components to streamline manufacturing and assembly of final parts while reducing supply chain costs.”
Nypro recently redesigned a medical display holder for a blood analysis laboratory instrument. By using additive manufacturing, engineers were able to consolidate 36 parts into six parts.
“This yielded a major savings in design, supply chain and production costs, while accelerating time to market,” says Forbes.
Another medical device manufacturer that’s bullish on additive manufacturing is Johnson & Johnson Inc. The company operates a 3D Printing Center of Excellence that specializes in developing new ways to produce devices such as customized surgical tools and knee implants.
“Through 3D printing technology, we can print exactly what the patient needs to replace the degraded bone,” says Sam Onukuri, a mechanical engineer who heads up the center. “The implant can be made based on a CT or MRI scan from thousands of miles away.”
In the past, when people had their knees replaced, doctors typically had an option of five or six implants of different sizes, and a set of surgical instruments to go with them. Finding a perfect match could be challenging, resulting in longer surgeries and recoveries.
“3D-printed implants based on patient scans can achieve an exact fit for the joint,” claims Onukuri. “In addition, the specific surgical tools needed for the surgery, which tend to be complex, can be printed.”
Additive manufacturing can also speed up the production of tools. “Surgical instruments have a lot of moving parts,” explains Onukuri. “Traditionally, [machine tools are] used to create individual components that go into a particular instrument, then you [assemble the instrument] with screwdriving or welding.
“[Today], we can print the entire instrument at one time,” Onukuri points out. “When the product comes out [of the printer], it is fully functional. It can really shrink down the process, and make it a lot faster and less expensive to manufacture.”
Onukuri and his colleagues are also working on printing tissues that can replace or augment damaged organs. “Bioprinted tissues or organs can be used for drug testing and screening, eliminating the need for humans or animals in clinical trials,” he points out.
“To make products now, we have large factories that require a significant investment,” says Joseph Sendra, global vice president for manufacturing, engineering and technology at Johnson & Johnson. “With 3D printing, we can potentially move manufacturing to a very small footprint, doing the same thing closer to the customer. That means products do not need to be shipped as far, and there’s a faster turnaround.
“Design for additive manufacturing is different than design for traditional manufacturing,” explains Sendra. “It gives you the ability to consider many more solutions than you had before. But, every engineer can’t think that way. We have to teach them that there’s a difference. They need to look at the problem from a new point of view.”
FDA Approval
Additive manufacturing got a shot in the arm recently when the U.S. Food and Drug Administration (FDA) issued a long-awaited report on the technology. That should pave the way for more production-ready printed parts.
“Solving medical certification and qualification issues is a complex area, and the FDA has become much more involved over the last two years,” says Scott Dunham, vice president of research at SmarTech Markets Publishing LLC, a market research firm that specializes in additive manufacturing.
“This has culminated in the release of an official guidance from the FDA for printed medical devices,” notes Dunham. “It seeks to help [manufacturers] understand what types of standards have to be met for clearances and use.
“For emergency surgeries, where a custom solution is ideally needed and could be used to save a life or drastically alter the quality of life for a patient, the regulatory pathway is much, much simpler,” adds Dunham. “Therefore, we have plenty of instances of patient-specific devices made via 3D printing. However, for more widespread use in scenarios outside of these somewhat rare cases, the regulatory pathway is more difficult.”
Many industry observers believe the FDA action will usher in a new era of printed medical devices and encourage more companies to invest in the technology. In December 2017, the agency issued a comprehensive technical framework to advise manufacturers creating medical products on 3D printers.
“The FDA is preparing for a significant wave of new technologies that are nearly certain to transform medical practice,” says Scott Gottlieb, M.D., commissioner of the FDA. “We’re working to provide a more comprehensive regulatory pathway that keeps pace with those advances, and helps facilitate efficient access to safe and effective innovations that are based on these technologies.
“The FDA has reviewed more than 100 devices currently on the market that were manufactured on 3D printers,” notes Gottlieb. “This is likely just the tip of the iceberg, given the exponential growth of innovative research in this field.
“We envision that burn patients in the near future will be treated with their own new skin cells that are 3D printed directly onto their burn wounds,” adds Gottlieb. “Further down the road, there is the potential for this same technology to eventually be used to develop replacement organs.”
Engineers in the Center for Devices and Radiological Health have been conducting research to investigate the effect of design changes on the safety and performance of printed medical devices, and to determine how iterative changes alter the devices’ fit and functionality.
The new FDA guidance aims to help advise medical device manufacturers on technical aspects of additive manufacturing, such as clarifying what the FDA recommends manufacturers include on submissions for 3D-printed medical devices. It includes advice on various approaches to 3D printing, such as device design, testing of products for function and durability, and quality system requirements.
“Overall, it will help manufacturers bring their innovations to market more efficiently by providing a transparent process for future submissions,” claims Gottlieb. “[It will ensure that] our regulatory approach is properly tailored to the unique opportunities and challenges posed by this promising new technology.
“But, this technical guidance…is only intended to provide the FDA’s initial thoughts on an emerging technology, with the understanding that our recommendations are likely to evolve as the technology develops in unexpected ways,” warns Gottlieb.
“To help ensure the safety and effectiveness of these products, we’re working to establish a regulatory framework for how we plan to apply existing laws and regulations that govern device manufacturing to nontraditional manufacturers,” says Gottlieb. “[This includes] medical facilities and academic institutions that create 3D-printed personalized devices for specific patients they are treating.”
"The agency succeeded in qualifying expectations to assess patient risks, and the guidance document reads very well," notes Watershed Idea Factory's Cordaro. "However, [medical device manufacturers] need to anticipate that this document, and other additive manufacturing standards, may continue to evolve."
Printing Processes
Many different additive manufacturing processes exist and new materials are continually being developed for medical applications. Each option has pros and cons that engineers must carefully consider.
Several additive printing methods can be used. Popular options include electron beam melting, fused deposition modeling (FDM) and stereolithography. There are also powder-bed systems, such as direct metal laser sintering and selective laser melting. And, there are powder-fed systems, such as directed energy deposition and laser metal deposition.
Each 3D printing technology is compatible with a different class of materials, which will have differing temperature resistance, tensile strength, elongation at break and chemical resistance. Medical device engineers need to match their application to their material, resolution and other requirements, and choose a technology accordingly.
A wide variety of printers can be used with different types of raw materials to produce parts and subassemblies made out of plastics such as ABS or nylon, and metals such as cobalt chrome or titanium. However, different processes and materials are limited in terms of print quality and how large of a part they can build.
Multimaterial printers, such as the Stratasys Connex3 Objet260, allow materials of different properties to be combined easily, greatly enhancing the ability to make more complex and functional devices.
"The ability to 3D print a myriad of materials is the key to the future," says Charlie Wood, a product designer at 3Form Design. "Currently, FDM can print in two materials, thanks to dual extruders with two nozzles, each using a different filament type."
Traditionally, plastic has been the most common raw material used for 3D printing, because of cost and versatility.
According to Nypro’s Forbes, extensive testing of various 3D printers has provided Jabil engineers with a variety of options for producing medical devices using plastic materials. The company was an early adopter of HP’s Multi Jet Fusion printers.
“The printers use PA-12 nylon polymer,” says Forbes. “Because the plastic materials are deployed in layers during the 3D-printing process, the HP printer is well suited for producing plastic devices with embedded printed electronics or sensors.
“In contrast, injection molding puts a lot of stress on electronics and sensors,” claims Forbes. “As a result, manufacturers of wearable devices must go to great lengths to protect the electronics during the production process.
“Current materials are limited and costly, so it’s imperative to develop new, economical materials to optimize specific production processes,” warns Forbes.
Suppliers are developing new materials to meet varying requirements for structural, cosmetic and reliability performance.
“There are [many] new materials and variations of materials being tested at additive manufacturing research centers,” says Watershed Idea Factory’s Cordaro. “However, it is really the advances in process controls that are allowing additive manufacturing to enter the medical device industry on a grander scale.”
“Most of the available plastics are aligned with materials like Nylon 11 and Nylon 12, as well as ABS, polyactic acid and polyetheretherketone,” adds SmarTech’s Dunham. “For a good portion of printed medical models, some form of UV-curable acrylate photopolymer resin is used. There are also a lot of models printed in plain old powdered polyamide 12.
“For things like surgical cutting guides, the material of choice is also generally polyamide 12, but this is mostly just because it is the most easily and widely sinterable powdered thermoplastic,” explains Dunham. “Dental surgical cutting guides, and some specialized craniomaxillofacial cutting guides, can be made with UV-curable acrylate or epoxy resins as well.”
When it comes to anything interacting inside the human body, such as knee implants, titanium is king. Plastics are rarely used.
However, Dunham says there is at least one FDA-approved spinal implant and cranial implant system made from polyetherketoneketone (PEKK). “For plastic printed implants in general, PEKK is likely to be the material of choice for the future, because of its great strength, biocompatibility and radioluminescence,” he points out.
Metal Mania
The newest trend in medical device additive manufacturing is metal printing.
“Metal printing will find more medical applications [in the near future] as the technology continues to advance and engineers learn the caveats of design for manufacturing,” predicts Gordon Styles, president and CEO of Star Rapid, a low-volume metal 3D printing company that specializes in direct metal laser melting.
“For internal medicine applications, the choices are limited to material that’s biocompatible,” says Styles. “Many plastics aren’t, but two common metal choices are: 316L stainless steel and titanium. These are ideal for load-bearing joints or maxillofacial replacement bones. And, if you’re going to use these metals, laser sintering or laser melting powder bed machines are the best option.
“The more widespread use of metal 3D printing creates new opportunities to build innovative medical devices, such as surgical equipment and prosthetic limbs,” Styles points out. “316L stainless steel, titanium and cobalt chrome are ideal.”
“Cobalt chrome alloys are particularly prevalent in dentistry, while various alloys of titanium are found in medical and dental implants,” adds Ed Littlewood, marketing manager for medical dental products at Renishaw plc, a leading supplier of metal additive manufacturing systems. “These include commercially pure titanium and titanium 6AL-4V. Tantalum is also being used in some implant applications, as are titanium-niobium alloys.”
Tantalum appeals to medical device engineers, because of its great strength and superior biocompatibility vs. titanium, which gained favor for its superior biocompatibility compared to cobalt chrome.
“Magnesium is one of the most interesting metals currently being researched, [because it] could be seen as a resorbable material,” says Littlewood. “This could be a real game changer for implants throughout the body.”
"The types of metals used depends on intracorporeal (internal) and extracorporeal (external) applications," adds 3Form Design's Wood. "Typically, stainless steel and grade 5 titanium [are used] for intracorporeal applications, [because they] do not cause foreign body reactions. For extracorporeal applications, foreign body reactions must also be considered, as some individuals react to certain metals when they are in prolonged contact with their skin."
Printing with metal is different than printing with plastic. For instance, processing parameters, such as time and temperature are different.
Since plastic has a lower melting point than most metals, temperature and power requirements are lower. That can result in lower operating costs over time.
According to Frank Medina, technology leader for additive manufacturing at EWI, there are three key differences between printing metal and plastic parts:
- Material properties. Metal properties are much higher than plastic properties and must meet casting and forging standards.
- Speed. Plastic machines are generally faster than metal systems. Plastics require less energy and can be done in thicker layers.
- Cost. Machine cost and cost per build hour is more expensive for metals, because the systems and materials are more expensive.
Several emerging materials could hold potential for additive manufacturing applications in the medical device industry.
“Printing of inorganic materials that are comparable to ceramics, such as hydroxyapatite, are an interesting area,” says SmarTech’s Dunham. “These materials tend to mimic human bone and can be bioabsorbed into the body by allowing bone to grow into the device or implant.”
However, many 3D printing issues and challenges still need to be addressed by medical device manufacturers.
“Regulating and standardizing additive manufacturing is the challenge of the decade of this industry,” says Greg Paulsen, director of applications engineering at Xometry Inc., a 3D-printing service company that works with manufacturers such as Medtronic. “With traditional milling or molding you just know that the material will display certain properties and be reliable, because it is extremely standardized over decades of work.
“Additive manufacturing is a build-to-build process, meaning that the same part between builds, even in the same machine, could have minor deviations,” explains Paulsen. “This is not inherently a bad thing. It’s just something that the industry is not used to.
“With current standards, it requires more effort on the product side to release a medical device with additive parts,” warns Paulsen. “That being said, the market is moving quickly and we are seeing a lot of [manufacturers] taking the leap into FDA approval. So, it’s a very exciting time.”
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!